Details of the Drug
General Information of Drug (ID: DMR9Q4Y)
| Drug Name |
2-(1H-indol-3-yl)-N,N-dimethylethanamine
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
N,N-Dimethyltryptamine; N,N-DIMETHYLTRYPTAMINE; DIMETHYLTRYPTAMINE; 61-50-7; 3-(2-Dimethylaminoethyl)indole; 2-(3-Indolyl)ethyldimethylamine; N,N-Dimethyl-1H-indole-3-ethylamine; 2-(1H-Indol-3-yl)-N,N-dimethylethanamine; 1H-Indole-3-ethanamine, N,N-dimethyl-; DMT (psychogenic); (psychogenic); UNII-WUB601BHAA; 3-[2-(dimethylamino)ethyl]indole; EINECS 200-508-4; NSC 63795; WUB601BHAA; N,N-dimethyl-1H-Indole-3-ethanamine; BRN 0138259; CHEMBL12420; DEA No 7435; CHEBI:28969; DMULVCHRPCFFGV-UHFFFAOYSA-N; (2-Indol-3-ylethyl)dimethylamine; Indole,; Dimethyltryptamine
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
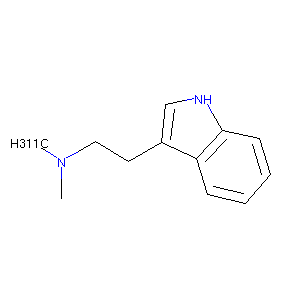 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 0 | Molecular Weight (mw) | 188.27 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | 2.5 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 3 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 1 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 1 | ||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
| Combinatorial Drugs (CBD) | Click to Jump to the Detailed CBD Information of This Drug | ||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 Drug Off-Target (DOT) |
|
|||||||||||||||||||||||||||||||||||||||||
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Discovery agent | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | N.A. | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
